Gemibine ® 1g Injection
(Gemcitabine Hydrochloride 1g)
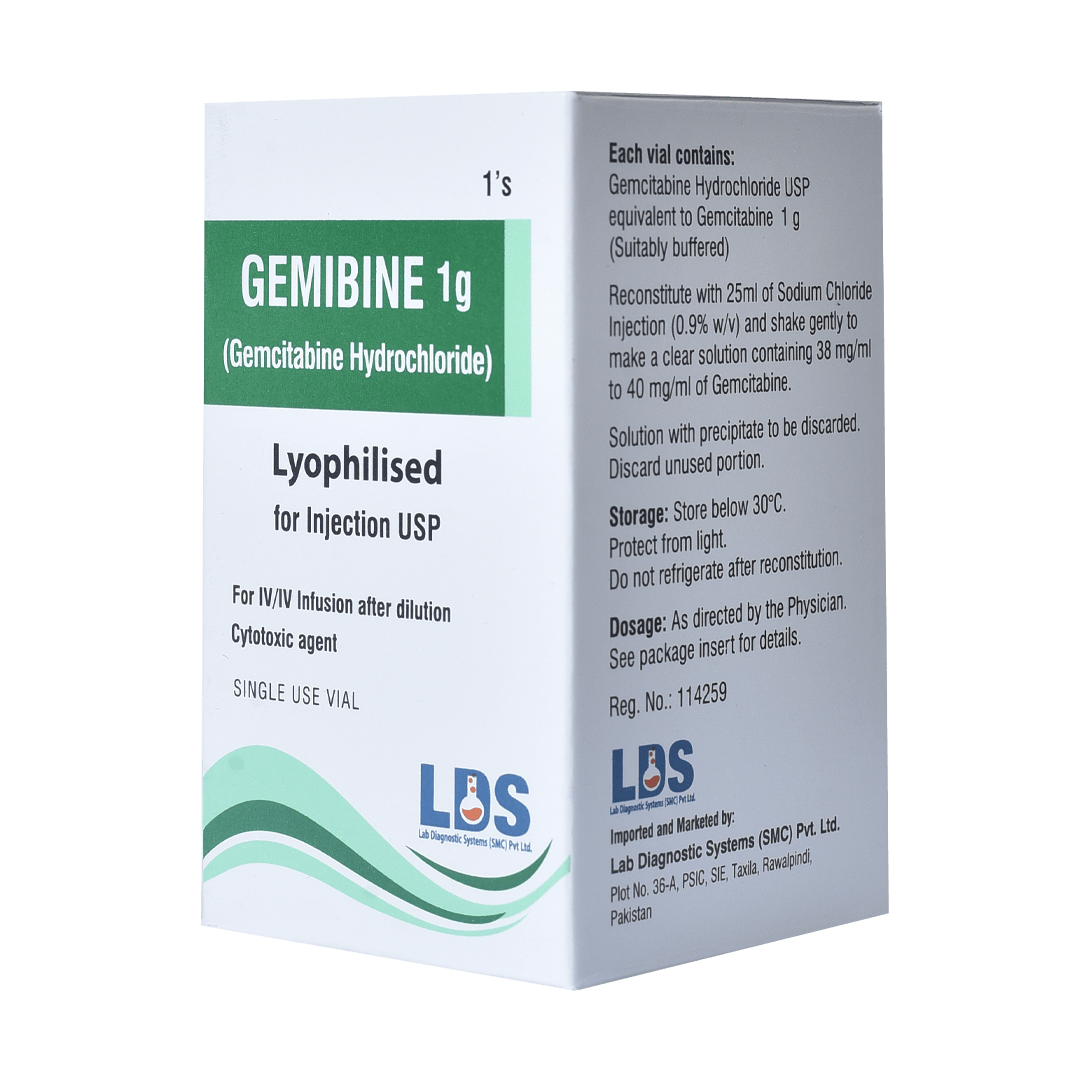
GENERIC NAME: Gemcitabine hydrochloride eq. to Gemcitabine 1g Injection
THERAPEUTIC CLASS: Nucleoside Metabolic Inhibitor
INDICATIONS & DOSAGE:
- In combination with carboplatin, for the treatment of advanced ovarian cancer that has relapsed at least 6 months after completion of platinum-based therapy
- In combination with paclitaxel, for first-line treatment of metastatic breast cancer after failure of prior anthracycline-containing adjuvant chemotherapy, unless anthracyclines were clinically contraindicated.
- In combination with cisplatin for the treatment of non-small cell lung cancer
- As a single agent for the treatment of pancreatic cancer.
STORAGE: Store below 30 °C. Protect from light.
PACK SIZE: 1×1’s
DOSAGE & ADMINISTRATION:
Gemcitabine Injection is for intravenous use only.
- Ovarian Cancer: 1000 mg/m2 over 30 minutes on Days 1 and 8 of each 21-day cycle
- Breast Cancer: 1250 mg/ m2 over 30 minutes on Days 1 and 8 of each 21-day cycle
- Non-Small Cell Lung Cancer: 1000 mg/ m2 over 30 minutes on Days 1, 8, and 15 of each 28-day cycle or 1250 mg/m2 over 30 minutes on Days 1 and 8 of each 21-day cycle.
- Pancreatic Cancer: 1000 mg/ m2 over 30 minutes once weekly for the first 7 weeks, then one-week rest, then once weekly for 3 weeks of each 28-day cycle
